Biological Hallmarks of Aging
The biological hallmarks of aging are a set of molecular and cellular damage processes that accumulate over time, leading to the functional decline and increased susceptibility to disease that characterize aging. These hallmarks were first described by Lopez-Otin et al. in 2016 and later expanded upon by Kennedy et al. in 2017. They can be categorized into nine primary hallmarks and four integrative hallmarks. Here, we describe the primary hallmarks of aging:

Telomere Shortening
Telomere shortening is a natural, age-related process that occurs in human cells due to several intrinsic and extrinsic factors. Telomeres are protective caps at the ends of our chromosomes that serve as a buffer against deterioration, much like the plastic tips on shoelaces. With each cell division, telomeres become shorter, eventually leading to cellular senescence or apoptosis (programmed cell death). This process is a significant driver of aging and age-related diseases. Here are some key reasons behind telomere shortening:
- The End Replication Problem: The primary reason for telomere shortening is the inability of DNA polymerase to fully replicate the lagging strand of the DNA double helix. This is known as the "end replication problem" or "replication problem." The enzyme responsible for DNA replication, DNA polymerase, cannot fully replicate the lagging strand due to its 5' to 3' directionality, leading to a loss of genetic material at the ends of the chromosomes, resulting in telomere shortening.
- Oxidative Stress: Reactive oxygen species (ROS) and oxidative stress are significant contributors to telomere shortening. ROS can damage telomeres directly, leading to their erosion. Moreover, oxidative stress can also impair the function of telomerase, an enzyme responsible for maintaining telomere length by adding DNA repeats to the ends of chromosomes.
- Inflammation: Chronic inflammation has been linked to accelerated telomere shortening. Inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), can inhibit telomerase activity and promote telomere attrition.
- Lifestyle Factors: Various lifestyle factors can contribute to telomere shortening. These include:
- Smoking: Smoking increases oxidative stress and inflammation, leading to accelerated telomere shortening.
- Poor Diet: A diet high in processed foods, sugars, and unhealthy fats can contribute to oxidative stress and inflammation, promoting telomere attrition.
- Physical Inactivity: Regular physical activity can help maintain telomere length, while a sedentary lifestyle can lead to telomere shortening.
- Stress: Chronic stress can activate the hypothalamic-pituitary-adrenal (HPA) axis, leading to increased cortisol levels, which in turn can promote telomere shortening.
- Environmental Factors: Exposure to environmental toxins, such as heavy metals, pesticides, and air pollution, can contribute to oxidative stress and inflammation, ultimately leading to telomere shortening.
To maintain telomere length and slow down aging, it is essential to adopt a healthy lifestyle that includes a balanced diet rich in fruits, vegetables, and whole foods, regular physical activity, stress management techniques, adequate sleep, and avoidance of environmental toxins. Additionally, exploring natural compounds and supplements that can support telomere health, such as astragalus, curcumin, resveratrol, and green tea catechins, may be beneficial.
Summary: Unraveling Telomere Shortening: Causes, Consequences, and Lifestyle Solutions
Stem Cell Exhaustion
Stem cell exhaustion, a phenomenon also known as stem cell depletion or stem cell senescence, is a critical aspect of aging and tissue homeostasis that has significant implications for health and longevity. It refers to the progressive loss of functional stem cells in various tissues and organs, leading to impaired tissue regeneration and repair capabilities. This process is characterized by the accumulation of senescent or exhausted stem cells that have lost their ability to self-renew and differentiate into specialized cell types.
The concept of stem cell exhaustion was first introduced in the context of hematopoiesis, the process by which blood cells are produced, and has since been observed in various tissues, including the skin, muscle, brain, and gut. The exhaustion of stem cells is thought to be a result of cumulative damage from oxidative stress, telomere shortening, and the accumulation of DNA damage, all of which are hallmarks of aging.
Stem cell exhaustion can be categorized into two main types:
- Intrinsic exhaustion: This type of exhaustion is caused by internal cellular changes, such as telomere shortening, DNA damage, and the accumulation of reactive oxygen species (ROS). These changes lead to a decline in the self-renewal capacity and differentiation potential of stem cells, ultimately resulting in their functional decline.
- Extrinsic exhaustion: This type of exhaustion is driven by signals from the stem cell niche, the microenvironment that supports and regulates stem cell function. Changes in the niche, such as inflammation, altered oxygen tension, and the accumulation of senescent cells, can negatively impact stem cell function and contribute to their exhaustion.
The consequences of stem cell exhaustion are far-reaching and impact various aspects of health and aging. Some of the key implications include:
- Impaired tissue regeneration and repair: As stem cells become exhausted, their ability to replace damaged or lost cells is compromised, leading to impaired tissue regeneration and repair. This can result in delayed wound healing, increased susceptibility to infections, and a reduced capacity to respond to tissue damage or injury.
- Accelerated aging: The progressive loss of functional stem cells contributes to the overall aging process, as tissues and organs become less able to maintain their function and structure. This can lead to a decline in organ function and an increased risk of age-related diseases.
- Increased cancer risk: While stem cell exhaustion is generally considered a protective mechanism against cancer, as it limits the number of divisions a cell can undergo, it can also contribute to cancer initiation and progression. Exhausted stem cells may accumulate mutations and become transformed, leading to the development of cancer stem cells and the initiation of tumors.
- Metabolic dysfunction: Stem cell exhaustion has been linked to metabolic dysfunction, as exhausted stem cells exhibit altered metabolic profiles and impaired metabolic flexibility. This can contribute to the development of metabolic disorders, such as type 2 diabetes and obesity.
To address stem cell exhaustion and its consequences, various strategies have been proposed, including:
- Targeting intrinsic factors: Strategies aimed at targeting intrinsic factors, such as telomere shortening and DNA damage, include telomerase activation, DNA repair therapies, and the use of senolytics, which selectively eliminate senescent cells.
- Modulating the stem cell niche: Approaches aimed at modulating the stem cell niche include targeting inflammation, altering oxygen tension, and promoting the clearance of senescent cells from the niche.
- Harnessing the power of natural compounds: Natural compounds, such as curcumin, resveratrol, and sulforaphane, have been shown to exert beneficial effects on stem cell function and may help to mitigate stem cell exhaustion. These compounds can be found in various foods and supplements, and their use may contribute to the maintenance of stem cell function and overall health.
In conclusion, stem cell exhaustion is a critical aspect of aging and tissue homeostasis that has significant implications for health and longevity. Understanding the mechanisms underlying stem cell exhaustion and developing strategies to mitigate its consequences are essential for promoting healthy aging and preventing age-related diseases.
Summary: Unraveling Stem Cell Exhaustion: A Key to Healthy Aging and Disease Prevention
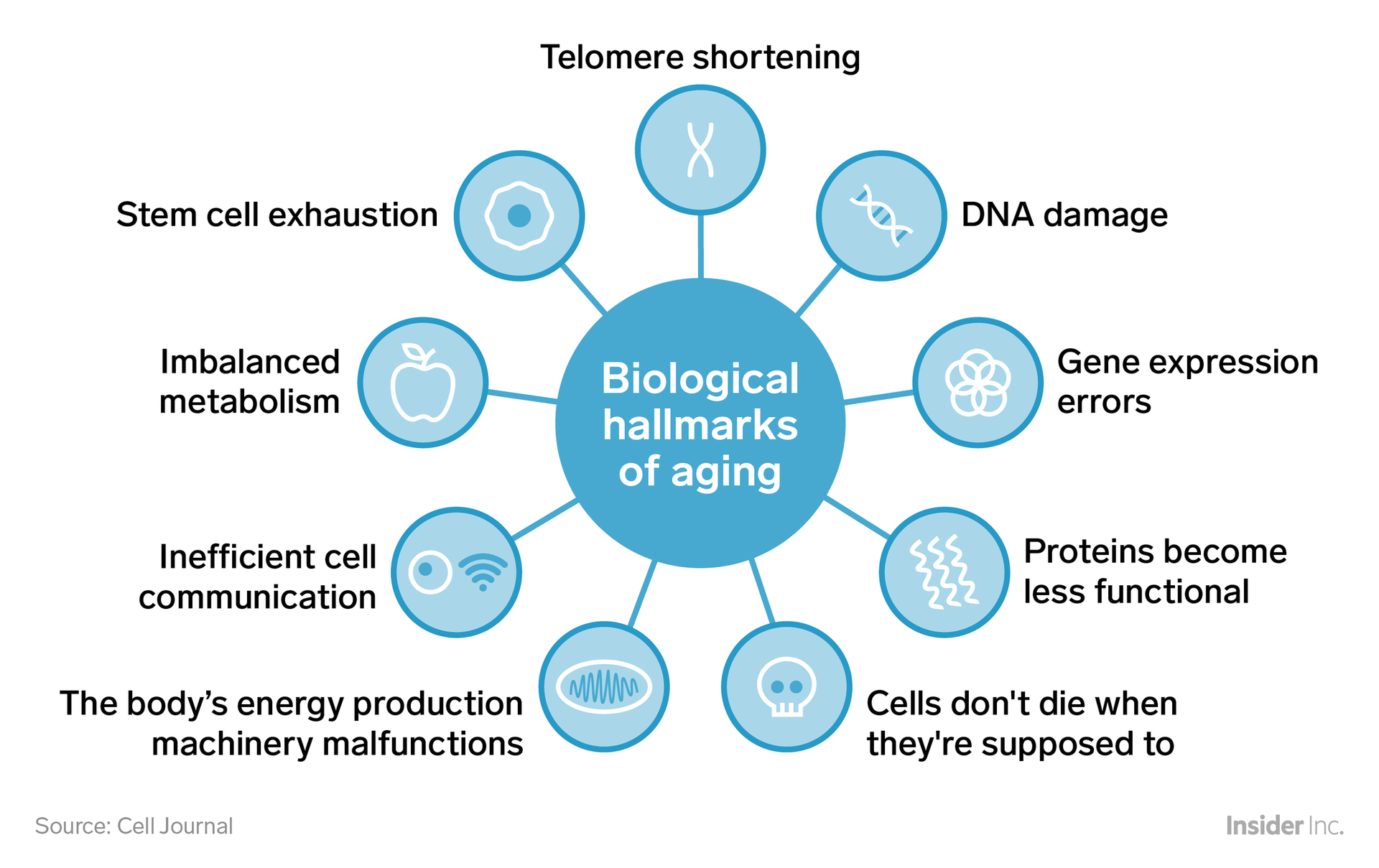
Imbalanced Metabolism
What is an imbalanced metabolism?
An imbalanced metabolism, also known as metabolic syndrome or dysmetabolism, refers to a group of medical disorders that affect how the body processes energy, resulting in an increased risk of various health issues. This condition is characterized by a combination of the following factors:
- Central Obesity: Excessive fat accumulation around the waist, often defined as a waist circumference of over 40 inches (102 cm) in men and over 35 inches (89 cm) in women.
- High Blood Pressure (Hypertension): Sustained elevated blood pressure, typically defined as a systolic blood pressure ≥130 mmHg or a diastolic blood pressure ≥85 mmHg.
- High Fasting Blood Sugar (Hyperglycemia): Elevated blood glucose levels, often indicative of insulin resistance or type 2 diabetes. A fasting blood glucose level of ≥100 mg/dL is considered high.
- High Triglycerides: Elevated levels of triglycerides, a type of fat in the blood. A triglyceride level of ≥150 mg/dL is considered high.
- Low HDL Cholesterol: Low levels of high-density lipoprotein (HDL) cholesterol, often referred to as "good" cholesterol. An HDL level of <40 mg/dL in men and <50 mg/dL in women is considered low.
To be diagnosed with metabolic syndrome, an individual must present with at least three of these five factors. The underlying causes of an imbalanced metabolism are complex and multifaceted, involving a combination of genetic predisposition, lifestyle factors, and environmental influences.
To address and manage an imbalanced metabolism, it is crucial to adopt a holistic approach that combines natural health strategies with lifestyle modifications. Here are some recommended actions:
- Nutrition: Adopt a balanced, whole-foods diet rich in fruits, vegetables, lean proteins, and healthy fats. Limit processed foods, sugars, and refined carbohydrates. Consider consulting with a natural health practitioner or a nutritionist to develop a personalized eating plan.
- Regular Exercise: Engage in regular physical activity, combining both cardiovascular exercises and strength training to improve insulin sensitivity and promote weight loss.
- Stress Management: Chronic stress can negatively impact metabolism. Incorporate stress- reduction techniques such as mindfulness, meditation, yoga, or other relaxation methods into your daily routine.
- Adequate Sleep: Prioritize getting enough quality sleep, as poor sleep can disrupt hormone balance and contribute to weight gain.
- Detoxification: Support your body's natural detoxification processes by consuming foods rich in antioxidants and phytonutrients, and consider periodic detoxification programs under the guidance of a qualified practitioner.
- Herbal Supplements: Consult with a natural health practitioner to explore the use of herbs and herbal extracts that may support healthy metabolism, such as bitter herbs, cinnamon, gymnema, or berberine.
Summary: Tackling Metabolic Imbalance: Causes, Symptoms, and Holistic Management Strategies
Inefficient cell communication
Inefficient cell communication, often referred to as cellular dysfunction, is a broad term that encompasses various impairments in the way cells interact with each other. This can lead to a wide range of health issues, as cells are the fundamental units of life, and their proper functioning is crucial for overall health and well-being. Here, we will delve into the causes, consequences, and natural strategies to improve cellular communication.
Causes of Inefficient Cell Communication:
- Poor Nutrition: Inadequate intake of essential nutrients, such as vitamins, minerals, and phytonutrients, can impair cellular communication. For instance, vitamin B3 (niacin) is crucial for maintaining healthy cell membranes and communication (1).
- Toxic Exposure: Exposure to environmental toxins, heavy metals, pesticides, and other harmful substances can disrupt cellular communication by damaging cell membranes, altering cellular signaling pathways, and inducing oxidative stress (2).
- Electromagnetic Pollution: Chronic exposure to electromagnetic fields (EMF) from devices like smartphones, Wi-Fi routers, and 5G towers can impair cellular communication by affecting calcium ion homeostasis and altering cell membrane fluidity (3).
- Inflammation: Chronic inflammation can lead to inefficient cell communication by activating stress responses, altering cellular signaling, and promoting cellular senescence (4).
- Glycation: The accumulation of advanced glycation end products (AGEs) due to high blood sugar levels can impair cellular communication by cross-linking cellular proteins and altering their function (5).
Consequences of Inefficient Cell Communication:
Inefficient cell communication can contribute to various health issues, including:
- Chronic degenerative diseases, such as cancer, diabetes, and cardiovascular disease (6)
- Neurodegenerative disorders, like Alzheimer's and Parkinson's disease (7)
- Autoimmune disorders, as impaired cellular communication can lead to miscommunication between immune cells (8)
- Accelerated aging and premature cellular senescence (9)
Natural Strategies to Improve Cellular Communication:
- Nutrition: Consume a balanced diet rich in whole foods, including fruits, vegetables, whole grains, lean proteins, and healthy fats. Incorporate superfoods, herbs, and herbal extracts that support cellular health, such as:
- Curcumin (turmeric) for its anti-inflammatory and antioxidant properties (10)
- Quercetin for its ability to modulate cellular signaling pathways (11)
- Resveratrol for its anti-inflammatory and antioxidant effects (12)
- Omega-3 fatty acids for their role in maintaining healthy cell membranes (13)
- Detoxification: Support your body's natural detoxification processes with a healthy lifestyle that includes regular exercise, adequate sleep, and stress management techniques. Consider incorporating detoxifying herbs, such as:
- Milk thistle (silymarin) to support liver function (14)
- Dandelion root to promote kidney function and detoxification (15)
- Activated charcoal to bind and eliminate toxins (16)
- Limit Exposure to Environmental Toxins: Reduce your exposure to environmental toxins by choosing organic foods, using natural personal care products, and creating a healthy living environment.
- Electromagnetic Protection: Limit your exposure to electromagnetic fields by using wired connections for devices, keeping a safe distance from EMF sources, and using EMF-blocking materials for protection.
- Manage Inflammation: Incorporate anti-inflammatory foods, herbs, and lifestyle practices to manage chronic inflammation, such as:
- Consuming a Mediterranean-style diet (17)
- Practicing stress-reducing activities, like yoga and meditation (18)
- Incorporating anti-inflammatory herbs, such as ginger, boswellia, and devil's claw (19)
Summary: Boosting Cellular Communication: Unraveling Causes, Consequences, and Natural Strategies
The body's energy production machinery malfunctions
The body's energy production machinery, also known as cellular respiration, is a complex process that converts the energy from food molecules into ATP (adenosine triphosphate), the body's primary energy currency. This process occurs in the mitochondria, the powerhouses of cells, and involves three main stages: glycolysis, the Krebs cycle, and the electron transport chain. Malfunctions in this machinery can lead to various health issues, ranging from fatigue and weakness to severe, life-threatening conditions. Here are some key malfunctions that can occur in the body's energy production machinery:
- Glycolysis Dysfunction:
- Pyruvate Dehydrogenase Complex (PDC) Deficiency: PDC is responsible for converting pyruvate (a product of glycolysis) into acetyl-CoA, which enters the Krebs cycle. Deficiencies in PDC enzymes can lead to lactic acidosis, a condition characterized by elevated lactate levels in the blood, causing muscle weakness, fatigue, and in severe cases, metabolic acidosis.
- Lactate Dehydrogenase (LDH) Deficiency: LDH catalyzes the conversion of pyruvate to lactate. Deficiencies in LDH can also result in lactic acidosis, with similar symptoms as PDC deficiency.
- Krebs Cycle Dysfunction:
- Krebs Cycle Enzyme Deficiencies: Deficiencies in enzymes like succinate dehydrogenase (SDH), fumarase, or malate dehydrogenase can lead to accumulation of intermediates, causing metabolic acidosis, encephalopathy, and developmental delays. These conditions are often inherited and can be life-threatening if left untreated.
- Fatty Acid Oxidation Disorders (FAOD): Although not directly part of the Krebs cycle, FAODs can impair energy production by affecting the supply of acetyl-CoA. FAODs can cause hypoketotic hypoglycemia, hepatomegaly, and Reye syndrome, a severe condition that can lead to liver failure and encephalopathy.
- Electron Transport Chain (ETC) Dysfunction:
- Mitochondrial Respiratory Chain Disorders (MRCD): MRCDs are caused by mutations in genes encoding ETC complexes or assembly factors. They can lead to various symptoms, including muscle weakness, exercise intolerance, developmental delays, seizures, and in severe cases, Leigh syndrome, a progressive neurodegenerative disorder.
- Coenzyme Q10 (CoQ10) Deficiency: CoQ10 is a crucial cofactor in the ETC. Deficiencies can cause muscle weakness, seizures, ataxia, and in some cases, dilated cardiomyopathy.
- Mitochondrial DNA (mtDNA) Mutations: Mutations in mtDNA can impair energy production by affecting mitochondrial function. mtDNA mutations can cause a wide range of symptoms, including muscle weakness, exercise intolerance, hearing loss, and neurological abnormalities.
To maintain optimal energy production and prevent malfunctions, it is essential to consume a balanced diet rich in whole foods, engage in regular physical activity, manage stress, and avoid toxins that can damage mitochondria. Additionally, addressing any underlying nutrient deficiencies, such as those in B vitamins, iron, or magnesium, can help support healthy energy production.
Summary: Malfunctions in the Body's Energy Production Machinery: Causes and Consequences
Cells don't die when they are suppose to
The phenomenon you're describing, where cells fail to die when they should, is a key aspect of aging and disease, particularly cancer. This is largely due to the dysregulation of programmed cell death, or apoptosis. Here are several factors contributing to this issue:
- Telomere shortening: Telomeres are protective caps at the ends of our chromosomes that shorten as cells divide. Once they reach a critically short length, the cell should undergo apoptosis. However, in some cases, cells can bypass this mechanism, leading to uncontrolled cell growth and potentially cancer. This can be influenced by lifestyle factors such as stress, poor diet, and exposure to toxins (Blasco, 2005).
- Oxidative stress: Reactive oxygen species (ROS) can damage cellular components, triggering apoptosis. However, chronic oxidative stress can lead to DNA damage and impaired apoptosis, allowing damaged cells to survive and proliferate (Fiers et al., 1999). Consuming antioxidants from natural sources like fruits, vegetables, and herbs can help mitigate this issue.
- Inflammation: Chronic inflammation can disrupt apoptosis signaling, promoting cell survival and contributing to cancer development (Balkwill & Mantovani, 2001). Reducing inflammation through diet, stress management, and natural anti-inflammatory agents can help restore proper cell death.
- Oncogenes and tumor suppressors: Mutations in oncogenes (e.g., RAS, MYC) and tumor suppressors (e.g., TP53, BRCA) can dysregulate apoptosis, leading to uncontrolled cell growth. Many natural compounds, such as curcumin, resveratrol, and genistein, have been shown to induce apoptosis in cancer cells by targeting these pathways (Shen et al., 2012).
- Environmental toxins: Exposure to chemicals like pesticides, herbicides, and heavy metals can disrupt apoptosis, contributing to various diseases (Eriksen et al., 2007). Detoxification strategies, such as consuming a clean diet and using natural detoxifying agents, can help mitigate this issue.
To promote healthy cell death and overall health, consider the following action steps:
- Consume a nutrient-dense diet rich in fruits, vegetables, and herbs to provide antioxidants, anti- inflammatory compounds, and natural apoptosis-inducing agents.
- Manage stress through techniques such as meditation, yoga, and regular exercise.
- Limit exposure to environmental toxins by choosing organic foods, using natural personal care products, and promoting a clean living environment.
- Consider supplementing with natural compounds like curcumin, resveratrol, or green tea extract to support healthy cell death.
- Regularly engage in activities that promote physical fitness and cardiovascular health.
Summary: Uncontrolled Cell Survival: The Root of Aging and Cancer
Proteins become less functional
In aging, proteins become less functional due to a combination of post-translational modifications, aggregation, and changes in cellular environment. These alterations lead to a decline in protein function, contributing to various age-related diseases and overall physiological decline. Here are some key factors contributing to reduced protein functionality in aging:
- Post-translational modifications (PTMs): As proteins age, they accumulate various PTMs, such as oxidation, glycation, and phosphorylation. These modifications can alter protein structure, affecting their function and stability. For instance:
- Oxidation: Reactive oxygen species (ROS) generated during aging can oxidize proteins, leading to the formation of protein carbonyls and other modified amino acids. This can disrupt protein folding, interfere with protein-protein interactions, and impair enzyme activity (Stadtman, 2006).
- Glycation: Non-enzymatic glycation occurs when reducing sugars, such as glucose, react with proteins to form advanced glycation end products (AGEs). AGEs can cross- link proteins, leading to increased stiffness and reduced functionality (Vlassara et al., 2016).
- Phosphorylation: Age-related changes in kinase and phosphatase activities can lead to abnormal phosphorylation patterns, affecting protein function and cellular signaling pathways (López-Otín et al., 2016).
- Protein aggregation: Aging is associated with the accumulation of protein aggregates, such as amyloid fibrils and other oligomers. These aggregates can be toxic to cells, disrupting cellular structures and impairing protein function. For example, amyloid-beta plaques and tau tangles are hallmarks of Alzheimer's disease, a neurodegenerative disorder linked to aging (Hardy & Selkoe, 2002).
- Changes in cellular environment: Aging is characterized by a decline in cellular maintenance processes, such as protein quality control systems (proteasomal and autophagic degradation) and protein synthesis. This can lead to the accumulation of damaged proteins and reduced production of functional proteins (López-Otín et al., 2016).
- Altered protein folding: Age-related changes in the cellular environment, such as altered redox status, pH, and ion concentrations, can affect protein folding and stability. This can lead to the accumulation of misfolded proteins and impaired protein function (Morimoto, 2008).
To maintain protein functionality and slow down aging, it is essential to adopt a healthy lifestyle that includes a balanced diet rich in antioxidants, regular exercise, adequate sleep, and stress management. Additionally, exploring natural compounds and therapies that target age-related protein modifications, such as senolytics and autophagy-inducing agents, may offer promising avenues for promoting healthy aging (Kirkland & Tchkonia, 2020).
Summary: Protein Dysfunction in Aging: Post-Translational Modifications, Aggregation, and Environmental Changes Drive Physiological Decline
Gene Expression Errors
Gene expression errors, also known as gene expression abnormalities or dysregulation, refer to
deviations from the normal pattern of gene expression, which is the process by which information from a gene is used to produce a functional gene product, such as a protein. These errors can occur at various stages of gene expression, including transcription, RNA processing, and translation, and can lead to a wide range of health issues, including genetic disorders, cancer, and other diseases.
Gene expression is a complex, tightly regulated process that is influenced by numerous factors, including DNA sequence, epigenetic modifications, transcription factors, and environmental cues. Errors in gene expression can arise from mutations in the DNA sequence, copy number variations, or epigenetic modifications that alter the accessibility of genes to transcription factors. Additionally, environmental factors such as toxins, infections, and stress can also disrupt normal gene expression patterns.
Gene expression errors can manifest in several ways, including:
- Overexpression: This occurs when a gene is expressed at higher levels than normal, leading to an excess of the corresponding protein. Overexpression can be caused by gene amplification, loss of negative regulatory elements, or activation of upstream signaling pathways. Overexpression of oncogenes, for example, is a common feature of many cancers.
- Underexpression: This refers to decreased expression of a gene, leading to reduced levels of the corresponding protein. Underexpression can result from gene deletions, mutations in regulatory sequences, or activation of repressor proteins. Underexpression of tumor suppressor genes, for instance, is a key event in the development of many cancers.
- Misexpression: This involves expression of a gene in the wrong cell type, at the wrong time, or in response to the wrong stimulus. Misexpression can be caused by mutations in regulatory sequences, changes in chromatin structure, or disruption of signaling pathways. Misexpression of genes can lead to developmental abnormalities, tissue homeostasis disruption, and cancer.
- Alternative splicing: This is a process by which a single gene can give rise to multiple mRNA transcripts, and consequently, multiple protein isoforms. Errors in alternative splicing can lead to the production of non-functional or dominant-negative protein isoforms, contributing to various diseases, including neurological disorders and cancer.
- The consequences of gene expression errors depend on the specific gene affected, the type of error, and the cellular context. Some gene expression errors are benign, while others can have severe consequences, such as genetic disorders or cancer. Understanding the mechanisms underlying gene expression errors is crucial for developing targeted therapies for various diseases.
Summary: Unraveling Gene Expression Errors: Causes, Consequences, and Therapeutic Implications
DNA Damage
DNA damage refers to alterations in the structure or information content of deoxyribonucleic acid (DNA), the genetic material present in the nucleus of cells. These alterations can occur due to various
endogenous and exogenous factors, leading to mutations that may have detrimental effects on cellular function and overall organism health. Here's a detailed overview of DNA damage, its causes, types, and consequences:
- Causes of DNA Damage:
- Endogenous Factors: These include errors during DNA replication, transcription, or repair; reactive oxygen species (ROS) generated by cellular metabolism; and DNA- damaging enzymes like topoisomerases.
- Exogenous Factors: These include environmental agents such as ultraviolet (UV) radiation, ionizing radiation, chemicals (e.g., carcinogens, pesticides), and viruses. Some common DNA-damaging agents are:
- Ultraviolet (UV) radiation: UV-B causes direct DNA damage, while UV-A generates ROS that can induce DNA damage.
- Ionizing radiation: Radiation causes DNA damage through the production of free radicals and direct energy transfer to DNA.
- Chemicals: Certain chemicals, such as polycyclic aromatic hydrocarbons (PAHs), aromatic amines, and nitrosamines, can form DNA adducts or cause DNA strand breaks.
- Viruses: Some viruses, like human papillomavirus (HPV), integrate their DNA into the host genome, causing damage and mutations.
- Types of DNA Damage:
- Base Damage: This includes modifications to the DNA bases, such as oxidation, alkylation, or deamination, leading to the formation of pyrimidine dimers (e.g., cyclobutane pyrimidine dimers and (6-4) photoproducts) upon UV exposure.
- Single-Strand Breaks (SSBs): These occur when one of the two sugar-phosphate backbones is broken, leaving a free 3'-OH and a 5'-phosphate group.
- Double-Strand Breaks (DSBs): These are more severe, as both sugar-phosphate backbones are broken, leading to the loss of both a 3'-OH and a 5'-phosphate group.
- Crosslinks: These occur when DNA is covalently linked to proteins or other DNA molecules, preventing replication and transcription.
- Chromosomal Aberrations: These include deletions, insertions, inversions, and translocations, which can lead to genetic instability and cancer.
- Consequences of DNA Damage:
- Cellular Level: DNA damage can lead to cell cycle arrest, apoptosis (programmed cell death), or, if left unrepaired, can cause mutations that may contribute to cancer development.
- Organismal Level: Accumulation of DNA damage and mutations can lead to various diseases, including cancer, neurodegenerative disorders, and premature aging.
- DNA Repair Mechanisms:
- Cells have evolved several DNA repair pathways to maintain genomic integrity, including base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR), double-strand break repair (DSBR), and direct reversal repair.
Summary: Unraveling DNA Damage: Causes, Types, Consequences, and Repair Mechanisms
Frequently Asked Questions (FAQs) on the Biological Hallmarks of Aging
What are the key categories of the biological hallmarks of aging?
The biological hallmarks of aging are a set of molecular and cellular damage processes that accumulate over time, leading to functional decline. They were first described in 2016 and can be broadly categorized into nine primary hallmarks and four integrative hallmarks. This article focuses on primary hallmarks, which include Telomere Shortening, DNA Damage, Gene Expression Errors, and Imbalanced Metabolism.
What causes telomere shortening, and how can it be mitigated?
Telomere shortening is a natural, age-related process that drives aging. Key causes include the "End Replication Problem" (the inability of DNA polymerase to fully replicate the lagging DNA strand), oxidative stress, and chronic inflammation. Lifestyle factors like smoking, poor diet, physical inactivity, and chronic stress also accelerate it6. Mitigation involves adopting a healthy lifestyle, including a diet rich in whole foods, regular physical activity, stress management, and exploring supplements like astragalus, curcumin, and resvera
How does "Stem Cell Exhaustion" impact tissue regeneration and cancer risk?
Stem cell exhaustion (or depletion) is the progressive loss of functional stem cells, resulting in impaired tissue regeneration and repair capabilities8888. This leads to:
- Impaired Tissue Repair: A reduced capacity to replace damaged or lost cells, leading to delayed wound healing and increased susceptibility to infections9.
- Increased Cancer Risk: Exhausted stem cells may accumulate mutations and become transformed, potentially leading to the development of cancer stem cells and tumor initiation10.
What are the clinical factors used to diagnose an "Imbalanced Metabolism" (Metabolic Syndrome)?
An imbalanced metabolism, or metabolic syndrome, refers to a group of disorders that affect energy processing11. An individual is diagnosed if they present with at least three of these five factors:
- Central Obesity (excessive waist fat) 12
- High Blood Pressure ($ge130/ge85$ mmHg) 13
- High Fasting Blood Sugar ($ge100~text{mg}/text{dL}$) 14
- High Triglycerides ($ge150~text{mg}/text{dL}$) 15151515
- Low HDL Cholesterol ($<40~text{mg}/text{dL}$ in men, $<50~text{mg}/text{dL}$ in women) 16
What happens when "Proteins become less functional" in aging?
Reduced protein functionality in aging is caused by the accumulation of post-translational modifications (PTMs), protein aggregation, and changes in the cellular environment17.
- PTMs: Modifications like oxidation (from ROS) and glycation (forming AGEs) alter protein structure, disrupting folding and impairing enzyme activity18181818.
- Aggregation: Aging is associated with the accumulation of toxic protein aggregates, such as the amyloid-beta plaques seen in Alzheimer's disease19191919.
- Cellular Environment: A decline in protein quality control systems (like proteasomal degradation) allows damaged proteins to accumulate20.

Dr. Michael Rudulph Maxon, AKA Johnny Delirious, Laboratory Naturopathic Doctor, gives expert advice rooted in holistic healing principles, drawing on 40 years of professional experience in the health industry. He helps his patients recover and heal using food and Ancient Greek therapies, utilizing organic remedies that are all backed by modern laboratory science. He is unquestionably the only TRUE Addiction & Hepatitis A, B, and C Recovery Pioneer. Free of mood-altering substances (cocaine) since 1991, with no viral load or antibodies of hepatitis since 1994, and no cirrhosis since 1995. Nobody in his life—including doctors, friends, and family—thought he would live past 1992; they all said he was going to die. But, Johnny chose life, not death, and learned how to heal his body, mind, and spirit by developing new protocols with natural therapies, including the thoughtful application of homeopathic remedies where appropriate. For over 20 years, he has helped many others recover, including professionals like doctors, dentists, and lawyers, who prefer alternative medicine over chemical drugs or surgery to address the same conditions that everyone said were hopeless.
Contact Johnny for a Hair Tissue Mineral Analysis (HTMA) to get the right diet, supplements, and expert advice, benefiting from his 30 years of experience in these specialized protocols.
United States - 972-825-7912
http://www.johnnydelirious.com


